Cervical Cancer

Disease Burden of Cervical Cancer in India
What is Cervical Cancer
Cervical cancer begins when normal cells of the cervix undergo changes leading to abnormal growth. At least half of sexually active people will have HPV at some point in their lives, but few women will get cervical cancer. Cervical cancer continues to be an important public health problem, being the second most common cancer among women in India and accounting for a quarter of the world's burden; even though preventable-by screening and treatment of precancerous lesions.
Although the rates have shown some decline, they are still markedly higher than in developed countries with established screening programs. Another unique feature of cervical cancer is its viral etiology. Since all cases of cervical cancer cannot be prevented by screening, these countries have sought to supplement the prevention effort by vaccinating young girls against infection with HPV virus, the necessary cause of cervical cancer.
Since the vaccine was introduced in 2006, over 175 million women have been vaccinated and 62 countries have included them in their national programs, including LMICs. Important bodies like US vaccine Adverse Event Reporting System (VAERS) agencies in UK, Europe, Australia, WHO etc are carrying out post marketing surveillance. Professional organisations like FIGO & IPV have put out statements on safety. Evaluation of thousands nay millions of girls in comprehensive meta-analysis have shown no increase in the risk of auto immune diseases, new onset problems, mortality, change in sociocultural attitudes etc that can be attributed to the vaccine. What has been documented is a decline in the HPV type 16 & 18 contained in the vaccine that are responsible for over 70% of cases of cervical cancer worldwide(85% of cases in India) along with reductions in high grade precancerous lesions, seen in populations in Australia & USA. The vaccines have shown to be safe, effective and highly immunogenic with potential to generate herd immunity and no indication yet for the need of a booster dose.
Screening will show its results here and now, vaccination in the future. There should be clear understanding that the two strategies are complementary to each other and are both required. In developing countries with good EPI infrastructure administering vaccines may be easier and more acceptable than establishing and promoting a culture of screening. Nevertheless both are required and no efforts should be spared to make girls and women aware of their rights to better health.
Undoubtedly, there will be improvements in the future. There will be better understanding of optimum doses, new vaccines will emerge, indigenous vaccines are being developed . For each year that will delay implementation of vaccination and screening thousands of women will pay the price with their lives. The time to act is now!
Cervical Cancer - It is preventable. It is treatable. It is time to eliminate it
WHO is working to elminate cervical cancer as a global public health problem.
The Problem - If we do not act, deaths from cervical cancer will rise by almost 50% by 2040
Each year, more than 300 000 women die of cervical cancer. More than half a million women are dianosed. Every minute, one woman is diagnosed. Cervical cancer is one of the greatest threats to women's health. Each death is a tragedy, and can be prevented. Most of these women are not diagnosed early enough, and lack access to life-saving treatment. Studies have shown that prevention and early treatment of cervical cancer are also highly cost-effective.
"Elimination of cervical cancer as a global health problem is within reach for all countries. We know what works, and we now need to scale up our actions to prevent and control this disease."
- Dr Princess Nono Simelela, Assistant Director-General for Family, Women, Children and Adolescents, WHO
What is WHO doing?
In May 2018, WHO Director-General, Dr Tedros Adhanom Ghebreyesus made a global call for action towards the elimination of cervical cancer. Currently, most women diagnosed with cervical cancer are diagnosed with advanced cancers, where opportunity for cure is small. This compounded by lack of access to life-saving treatment in settings where the burden and need is highest.
Now is the time for global elimination of cervical cancer.
Join us to eradicate cervical cancer in India. Do contact us at www.beautifultomorrow.org.in or madhu.1950@yahoo.com
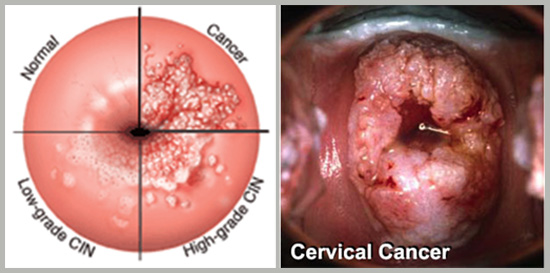
What does Cervical Cancer look like?
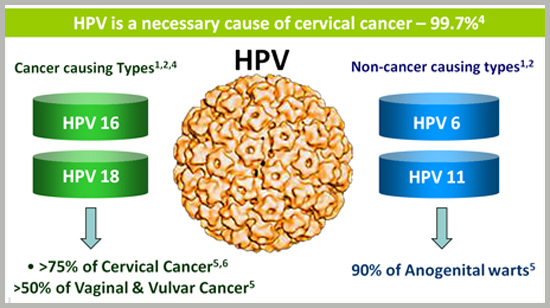
What causes Cervical Cancer?
Who is at risk of cervical cancer?
How HPV is transmitted?
What are the signs of cervical cancer?
Cervical Cancer Screening
Screening of malignancies of female genital tract and breast is necessary for their early detection and hence better management, good prognosis and better survival. India has the largest burden of cervical cancer in the world with an incidence of 5-30/100,000 females. Cervical cancer is a preventable disease by early detection through screening and primary prevention by vaccination. Screening tests available are Visual inspection with acetic acid (via), Visual inspection with Lugol's iodine (VILI), cervical cytology through Pap smear and liquid based cytology or by HPV DNA testing. The screening schedules recommended by ACOG states that women aged 25-65 years should preferably undergo combined screening with cytology and HPV DNA testing every 5 years (co-testing). Cytology alone every 3 years is also acceptable. However, screening by HPV testing alone is not recommended. The screening recommendations are same in women vaccinated against HPV. Primary prevention is available through vaccination which is a quadrivalent vaccine (Guada-sil) or bivalent vaccine (Cervarix) given at the dosing schedules of 0,1,and 6 months.
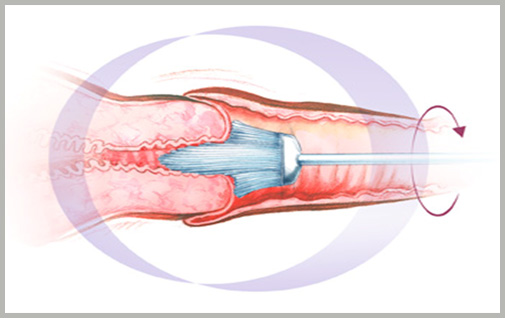
How is Cervical Cancer screened?
What exactly is done during screening?
Pap Smear Test
Cervical Cancer Vaccination
Why is Cervical Cancer important to you?

Endometrial Cancer Screening
Carcinoma endometrium rarely presents in the later stages as menstrual dysfunction and no screening strategy is universally adopted except in the high risk groups of hereditary cancers where annual endometrial biopsy is recommended from 35 years of age.
Ovarian Cancer Screening
No definitive tests is available for carcinoma ovary and multimodal screening with tumor marhers (CA-125, HE4) and transvaginal scan is recommended for high risk groups. Women with BRCA1 and 2 mutations should undergo prophylactic oophorectomy once child bearing is complete.
